Calcium sulfate is not very soluble in water either and accumulates as sludge at the marble acid interface inhibiting the reaction.
Marble chips and nitric acid.
Again i had to ensure that this was a fair test so i kept other factors constant throughout the experiment.
The reason i think it would vary is that you get different reactions when one puts pure copper in either concentrated or dilute nitric acid.
Marble is calcium carbonate which is not appreciably soluble in water.
Marble chips are mostly made up of calcium carbonate which is a alkaline compound.
Being alkaline it reacts with hydrochloric acid to produce calcium chloride water and carbon dioxide.
An investigation into how changing one variable influences the rate of reaction between marble chips and dilute hydrochloric acid planning section when dilute hydrochloric acid reacts with marble chips the following reactions occurs.
Caco3 2hno3 ca no3 2 co2 h2o this particular reaction is rather slow and so needs to be quicker by changing the factors that affect the rate of reaction.
An outline of an experiment that could be used to find the time and hence rate of reaction of marble chips and hydrochloric acid.
The results are shown below.
A conical flask contains the marble chips hydrochloric acid and the water that will make the reaction.
Aim the experiment will be on the reaction of nitric acid and marble chips.
Caco3 2hcl h2o co2 this is the reaction we will be investigating.
The product of marble s reaction with sulfuric acid is calcium sulfate.
In the investigation i am going to find out how the surface area affects the rate of reaction by measuring the amount of gas produced and weight loss in a reaction between small large pieces of marble chips calcium carbonate and hydrochloric acid per minute.
A tube to connect the conical flask to the measuring cylinder.
One for concentrated hno3 has no2 nasty nitrogen dioxide as a product while the other for dilute hno3 has not as nasty nitrogen monoxide no as a product.
I used 20cm3 of 2 molar nitric acid and 2 grams of marble chips and then i monitored the change in weight for 4 minutes.
Acids therefore must act on the surface of the solid marble chunks.
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
The marble chip is calcium carbonate caco3.
Investigating the rate of reaction between marble chips calcium carbonate and hydrochloric acid aim.
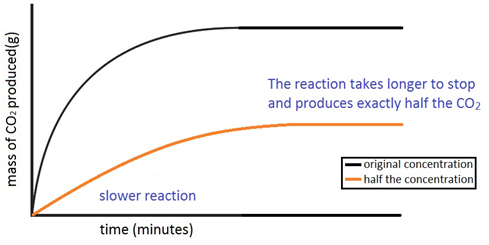
The rate of this reaction can be changed by changing the size of the marble chips.